Titanium oxide, commonly referred to as titanium dioxide (TiO2), is a naturally occurring compound widely recognized for its versatility and extensive industrial applications. As a critical material in various sectors, including paints, coatings, cosmetics, and environmental solutions, titanium oxide plays an indispensable role in modern manufacturing and technology. This article provides a detailed exploration of titanium oxide, covering its chemical and physical properties, production methods, applications, and safety considerations. With a focus on technical depth and practical insights, this guide aims to serve as a valuable resource for professionals, researchers, and enthusiasts seeking to understand the significance of this remarkable compound.
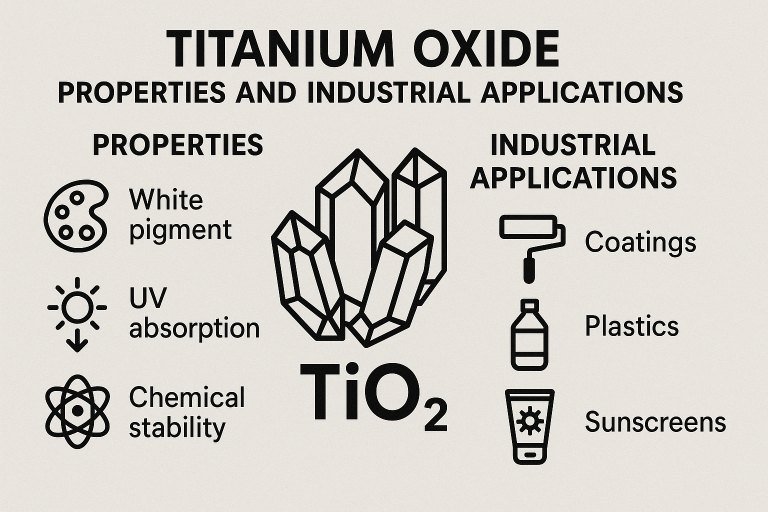
Chemical and Physical Properties of Titanium Oxide
Titanium oxide, with the chemical formula TiO2, exists primarily in three crystalline forms: rutile, anatase, and brookite. Among these, rutile and anatase are the most industrially relevant due to their stability and unique properties. Rutile is the most stable form at high temperatures, while anatase exhibits higher photocatalytic activity, making it suitable for specific applications like environmental purification.
The compound is characterized by its high refractive index, which ranges from 2.488 to 2.609 for anatase and 2.583 to 2.903 for rutile, depending on the wavelength of light. This property makes titanium oxide an excellent pigment, providing exceptional opacity and brightness in products like paints and coatings. Additionally, TiO2 is insoluble in water and most organic solvents, with a melting point of approximately 1,843°C and a boiling point of around 2,972°C. Its density varies slightly between forms, with rutile at 4.23 g/cm³ and anatase at 3.89 g/cm³.
Another notable property is its photocatalytic behavior, particularly in the anatase form, where exposure to ultraviolet (UV) light triggers reactions that can break down organic pollutants. This characteristic underpins its use in air and water purification systems. Furthermore, titanium oxide is non-toxic in most forms, which enhances its suitability for applications in food and cosmetics industries, provided it meets specific purity standards.
Production Methods of Titanium Oxide
The industrial production of titanium oxide primarily involves two processes: the sulfate process and the chloride process. Both methods aim to extract TiO2 from titanium-bearing ores such as ilmenite (FeTiO3) or rutile ore, with the choice of process depending on the raw material availability, cost, and desired purity of the final product.
In the sulfate process, ilmenite ore is treated with sulfuric acid to produce titanium sulfate, which is then hydrolyzed to form hydrated titanium dioxide. This intermediate is calcined at temperatures between 800°C and 1,000°C to yield the final TiO2 product. The sulfate process is often preferred for its ability to handle lower-grade ores, though it generates significant waste byproducts, including iron sulfate and acidic residues, which require careful management.
The chloride process, on the other hand, involves reacting rutile ore with chlorine gas and carbon at high temperatures (around 900°C to 1,000°C) to form titanium tetrachloride (TiCl4). This compound is then oxidized with oxygen at temperatures exceeding 1,200°C to produce pure TiO2 and regenerate chlorine gas for reuse. The chloride process is more environmentally friendly due to fewer waste products and is typically used for producing high-purity titanium oxide for specialized applications.
Both processes allow for the production of TiO2 in various particle sizes and surface treatments, tailoring the material for specific uses. For instance, pigment-grade TiO2 often undergoes surface coating with silica or alumina to enhance dispersibility and durability in paints and coatings.
Industrial Applications of Titanium Oxide
Titanium oxide is a cornerstone material in numerous industries due to its unique combination of properties. Below are some of the primary applications where TiO2 demonstrates its value and versatility.
Pigments in Paints and Coatings: Approximately 60% of globally produced titanium oxide is used as a pigment in paints, coatings, and plastics. Its high refractive index and opacity provide excellent hiding power, making it ideal for achieving bright, durable finishes. TiO2 is often incorporated at concentrations of 20-30% by weight in architectural paints to ensure coverage and resistance to weathering.
Cosmetics and Sunscreens: In the cosmetics industry, titanium oxide serves as a pigment and UV filter in products like sunscreens and foundations. Its ability to reflect and scatter UV radiation makes it effective in protecting skin from harmful rays, with particle sizes typically ranging from 10 to 50 nanometers for optimal UV blocking without visible whitening on the skin.
Photocatalytic Applications: The photocatalytic properties of anatase TiO2 are harnessed in environmental applications, such as air and water purification. When exposed to UV light, TiO2 generates reactive oxygen species that decompose organic pollutants, with reaction rates often measured in terms of pollutant degradation per hour under specific light intensities (e.g., 10-20 mg/L of pollutant degraded per hour under 365 nm UV light).
Food Industry: Titanium oxide is used as a food additive (E171) to enhance the whiteness and brightness of products like candies and chewing gum. Its use is strictly regulated, with purity levels required to exceed 99% and heavy metal content limited to less than 10 ppm to ensure safety for human consumption.
Other Uses: TiO2 also finds applications in ceramics, paper production, and as a catalyst support in chemical reactions. Its thermal stability and chemical inertness make it suitable for high-temperature processes and harsh environments.
Safety and Environmental Considerations
While titanium oxide is generally considered safe for most applications, certain considerations must be addressed to ensure its safe handling and use. In its bulk form, TiO2 is non-toxic and inert, posing minimal risk to human health. However, when used in nanoparticle form, particularly in sunscreens or inhalable dust, there are concerns about potential health effects due to its small particle size, which may penetrate biological barriers.
Regulatory bodies such as the European Food Safety Authority (EFSA) and the U.S. Food and Drug Administration (FDA) have established guidelines for TiO2 use in food and cosmetics, specifying acceptable daily intake levels and particle size restrictions. For instance, in food applications, the maximum allowable concentration of TiO2 as an additive is often capped at 1% by weight of the final product in certain regions.
From an environmental perspective, the production of titanium oxide, especially via the sulfate process, can generate significant waste, including acidic effluents and metal sulfates. Modern production facilities often implement waste treatment and recycling systems to minimize environmental impact, with some achieving up to 90% recovery of sulfuric acid in the sulfate process.
Additionally, the photocatalytic activity of TiO2, while beneficial for pollution control, requires careful management to prevent unintended degradation of materials in contact with TiO2-coated surfaces under UV exposure. This necessitates the use of protective coatings or stabilizers in certain applications to mitigate such effects.
Comparison of Titanium Oxide Forms
The following table provides a concise comparison of the two primary forms of titanium oxide, rutile and anatase, highlighting their key differences in properties and applications.
| Property | Rutile | Anatase |
|---|---|---|
| Crystal Structure | Tetragonal (more stable) | Tetragonal (less stable) |
| Density (g/cm³) | 4.23 | 3.89 |
| Refractive Index | 2.583-2.903 | 2.488-2.609 |
| Primary Application | Pigments, coatings | Photocatalysis, purification |
| Photocatalytic Activity | Low | High |
Conclusion
Titanium oxide (TiO2) stands as a pivotal material in modern industry, offering a unique blend of optical, chemical, and physical properties that cater to a wide range of applications. From its role as a high-performance pigment in paints to its photocatalytic capabilities in environmental solutions, TiO2 continues to demonstrate its indispensability. Understanding its properties, production methods, and safety considerations is essential for leveraging its full potential while addressing environmental and health concerns. This comprehensive overview serves as a foundation for further exploration and application of titanium oxide in diverse fields, ensuring its benefits are realized responsibly and effectively.