Aluminum oxide, commonly referred to as alumina, is a widely recognized chemical compound with the formula Al₂O₃. It is a naturally occurring mineral and an essential material in numerous industrial and commercial applications due to its exceptional physical and chemical properties. This article provides an in-depth exploration of the aluminum oxide compound, covering its composition, characteristics, production methods, and diverse uses across multiple sectors. With a focus on technical details and systematic analysis, this guide aims to serve as a valuable resource for professionals, researchers, and anyone seeking to understand this critical material.
Aluminum oxide is celebrated for its durability, resistance to corrosion, and high thermal stability, making it indispensable in industries ranging from ceramics to electronics. By delving into its properties and applications, we aim to present a thorough understanding of why this compound holds such prominence in modern technology and manufacturing.
Composition and Structure of Aluminum Oxide Compound
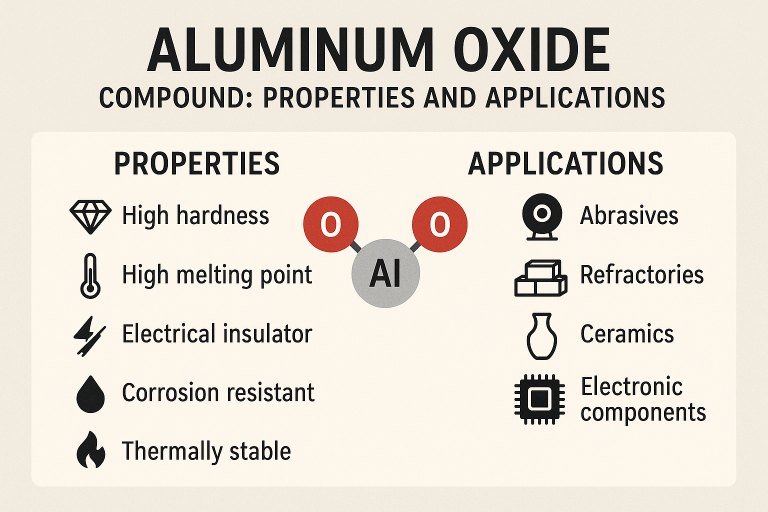
Aluminum oxide (Al₂O₃) is composed of two aluminum atoms and three oxygen atoms, forming a stable ionic compound. It exists in several crystalline forms, with the most common being alpha-alumina, which exhibits a trigonal crystal structure. This structure contributes to its remarkable hardness and thermal stability, positioning it as one of the most robust materials known. The compound's molecular weight is 101.96 g/mol, and it has a high melting point of approximately 2,072°C (3,762°F) and a boiling point of around 2,977°C (5,391°F).
The atomic arrangement in aluminum oxide results in strong ionic bonds, which are responsible for its high resistance to chemical reactions and mechanical stress. This structural integrity allows aluminum oxide to perform exceptionally well in extreme conditions, such as high temperatures and abrasive environments. Additionally, its polymorphic nature means it can exist in other forms like gamma-alumina, often used as a catalyst due to its high surface area.
Physical and Chemical Properties of Aluminum Oxide
Aluminum oxide is renowned for its impressive physical and chemical properties, which make it suitable for a wide range of applications. Below is a detailed examination of its key characteristics:
- Hardness: Aluminum oxide ranks 9 on the Mohs hardness scale, making it one of the hardest naturally occurring materials, just below diamond. This property is critical for its use in abrasives and cutting tools.
- Thermal Stability: With a melting point exceeding 2,000°C, aluminum oxide can withstand extreme temperatures without degrading, ideal for refractory materials in furnaces and kilns.
- Electrical Insulation: It is an excellent electrical insulator with a dielectric strength of approximately 13.4 kV/mm, widely used in electronic components and insulators.
- Chemical Inertness: Aluminum oxide is highly resistant to acids and alkalis at room temperature, ensuring durability in corrosive environments.
- Density: Its density is about 3.95 g/cm³, contributing to its use in lightweight yet strong composite materials.
These properties collectively make aluminum oxide a versatile compound, capable of meeting the stringent demands of various industrial processes. Its low thermal conductivity (approximately 30 W/m·K for alpha-alumina) also enhances its suitability for thermal barrier coatings.
Production Methods of Aluminum Oxide Compound
The primary method for producing aluminum oxide on an industrial scale is the Bayer process, which extracts alumina from bauxite ore. This process involves several key steps:
- Bauxite ore is crushed and mixed with sodium hydroxide under high pressure and temperature to form sodium aluminate.
- Impurities are filtered out, leaving a solution of sodium aluminate.
- Aluminum hydroxide is precipitated from the solution by cooling and seeding with aluminum hydroxide crystals.
- The precipitated aluminum hydroxide is calcined at high temperatures (around 1,100°C) to produce pure aluminum oxide.
Other methods, such as the Hall-Héroult process, are used to further refine alumina into aluminum metal, but the Bayer process remains the cornerstone for alumina production. The purity of the resulting aluminum oxide can reach up to 99.99%, depending on the intended application, with specific grades tailored for ceramics, abrasives, or catalysts.
Alternative production techniques, such as hydrothermal synthesis, are employed for specialized applications requiring specific particle sizes or morphologies. These methods ensure that aluminum oxide meets the precise requirements of advanced technologies.
Applications of Aluminum Oxide Across Industries
Aluminum oxide’s unique combination of properties enables its use in a wide array of industries. Below, we explore some of the most significant applications:
Abrasives and Cutting Tools
Due to its exceptional hardness, aluminum oxide is a primary material in the production of abrasives, including sandpaper, grinding wheels, and cutting discs. It is particularly effective for grinding and polishing metals, ceramics, and composites, offering superior performance and longevity compared to other abrasive materials.
Refractory Materials
In high-temperature environments, such as steelmaking and glass production, aluminum oxide serves as a key component in refractory bricks and linings. Its ability to withstand temperatures above 2,000°C without deformation ensures the integrity of industrial furnaces and kilns.
Electronics and Insulators
Aluminum oxide is widely used as an insulating material in electronic devices due to its high dielectric strength and thermal stability. It is a critical component in ceramic substrates, spark plug insulators, and integrated circuit packaging, ensuring reliable performance in demanding conditions.
Catalysis
Gamma-alumina, a form of aluminum oxide with a high surface area, is extensively used as a catalyst or catalyst support in chemical reactions. It plays a vital role in processes like petroleum refining and the production of synthetic materials, enhancing reaction efficiency.
Biomedical Applications
In the medical field, aluminum oxide is utilized in the manufacture of dental implants and orthopedic prosthetics due to its biocompatibility and wear resistance. Its inert nature ensures minimal reaction with bodily fluids, making it a safe choice for long-term implants.
Technical Specifications of Aluminum Oxide
For a clearer understanding of aluminum oxide’s capabilities, the following table summarizes its key technical parameters:
| Property | Value |
|---|---|
| Chemical Formula | Al₂O₃ |
| Molecular Weight | 101.96 g/mol |
| Melting Point | 2,072°C (3,762°F) |
| Boiling Point | 2,977°C (5,391°F) |
| Density | 3.95 g/cm³ |
| Hardness (Mohs Scale) | 9 |
| Thermal Conductivity | 30 W/m·K |
These specifications highlight why aluminum oxide is a preferred material in applications requiring durability, thermal resistance, and mechanical strength. The data can guide engineers and manufacturers in selecting the appropriate grade of alumina for their specific needs.
Environmental and Safety Considerations
While aluminum oxide is generally considered safe and non-toxic, certain precautions are necessary during its handling and processing. Fine alumina dust, if inhaled in large quantities, can pose respiratory risks, necessitating the use of protective equipment in industrial settings. Additionally, the production of aluminum oxide via the Bayer process generates red mud, a byproduct that requires careful management to prevent environmental contamination.
Regulatory guidelines, such as those from the Occupational Safety and Health Administration (OSHA), recommend exposure limits for alumina dust to ensure worker safety. Proper waste disposal and recycling practices are also critical to minimizing the environmental impact of alumina production.
Conclusion
Aluminum oxide compound stands as a cornerstone material in modern industry, offering unparalleled hardness, thermal stability, and chemical inertness. From abrasives and refractories to electronics and biomedical applications, its versatility is evident across numerous fields. By understanding its composition, properties, production methods, and applications, stakeholders can leverage aluminum oxide to meet the demands of high-performance environments.
This comprehensive guide has aimed to provide a detailed and systematic overview of aluminum oxide, emphasizing its technical attributes and practical significance. Whether you are a manufacturer, researcher, or engineer, the insights provided here can inform your approach to utilizing this remarkable compound in your projects.